Published Articles
A well-written and well-implemented quality risk management plan is an integral element of an effective quality system. During the development of your total quality strategy, the bottom line is that things can and will go wrong. Your QA management team and QMS system need to be designed with data quality management in mind. Your team should include employees from multiple functions who understand both risk and compliance.
Quality Risk Management
 The purpose of quality risk management is to help ensure continued compliance with regulatory requirements, such as good manufacturing practices. This is critical for risks analysis when events occur during manufacturing that can impact patient safety and product quality.
The purpose of quality risk management is to help ensure continued compliance with regulatory requirements, such as good manufacturing practices. This is critical for risks analysis when events occur during manufacturing that can impact patient safety and product quality.
ICH Q9
The International Council for Harmonization (ICH) Q9 states;
“Two primary principles of quality risk management are: the evaluation of the risk to quality should be based on scientific knowledge and ultimately link to the protection of the patient; and, the level of effort, formality, and documentation of the quality risk management process should be commensurate with the level of risk”.
In developing your company’s plan, you will need to consider key risk indicators and how to mitigate risk. Managing your company’s risk with a well-defined plan may help reduce strategic risk associated with poor ISO 9001 Standards. Most importantly, reducing inefficiencies associated with the product and the process is critical to improving quality.
A reduction in deviations/investigations, FDA warning letters, customer complaints, and product yield all improve the culture of quality.
Risk Management Framework
A risk management framework is used to evaluate all aspects of the manufacturing process and identify areas of vulnerability. These vulnerabilities need to be assessed for their financial risk impact on the operation and the potential level of risk they pose. Risk management in healthcare requires a holistic viewpoint from multiple departments across the enterprise.
Quality Risk Management Plan
A well-written quality risk management plan is an ongoing process requiring risk control documentation throughout the product lifecycle. It provides a solid risk management process for how to improve efficiency and minimize operational risk. Focus your operational risk management on the important activities to improve product quality rather than low-risk activities that have little impact. These are four key elements to consider when defining your risk mitigation strategies:
Element One: Gap Analysis
The first element is to perform an analysis of the identified risk associated with the operations. For example, consider project risk management if your product is being produced using an older manufacturing line. There is compliance risk that an out of date manufacturing process will experience breakdowns.
Element Two: Risk Evaluation
The second step is to evaluate the risk in terms of its impact on your ability to supply a quality product. In this case, frequent shutdowns can lead to product rejections, yield loss, and potential drug shortages. Search for risk management solutions that anticipate these types of supply chain risk in advance.
Element Three: Identify Controls
Once the risk has been identified and the impact evaluated, risk controls can help mitigate. For example, cybersecurity risk is a growing problem where manufacturing automation needs to be reviewed. Some of the possible mitigation control strategies might include conducting threat modeling to identify your risk in security. Ensure your risk mitigation plan also qualifies cyber risk management and how a cyber secure manufacturing line holistically prevents outside hacking.
Element Four: Data Management
The last key element needed is data input and management. Any risk management tool should be able to indicate if and when you need to employ one of your risk control strategies. Simple risk identification includes if you see an increase in downtime on the line or a steady decrease in yield. This data could be indicators that the manufacturing line is headed for a catastrophic failure and steps need to be taken to prevent a drug shortage situation.
Security Risk Management
The above discussion is only an example of a risk assessment in one area of an operation. Other areas of the process need to be evaluated for potential vulnerabilities and risk. These areas include an evaluation of the reliability of raw material suppliers, stability, and contractual supplier compliance, age and reliability of laboratory test equipment, etc. Supplier compliance also includes partnering with contract manufacturing organizations and contract test organizations.
Integrated Risk Management
A dynamic quality risk management plan will integrate the overall organization and identify high risk vulnerabilities. It will be proactive in identifying strategies for mitigation of the high-risk vulnerabilities. Data will be leveraged to perform continuous monitoring of the vulnerabilities. And, of course, the plan will provide the appropriate documentation and rationale for risk management programs.
Risk Management Consultant
Implementing a quality risk management plan in an organization can also be challenging. A risk management consultant can provide all applicable function personnel involved in the operations additional feedback. These functions include finance, manufacturing, regulatory affairs, purchasing, auditing, and senior management. The plan should be dynamic and should be modified as situations change.
Single-Source Vendor Risk
Let’s say you produce a product and you have a single-source supplier for one of your excipients. You have audited the supplier and have identified some significant gaps in their quality system. You identify this vulnerability in your quality risk management plan as a high-risk item because of the lack of vendor compliance.
One of your mitigation strategies might be to qualify an alternate supplier for the excipient. Once you have qualified that alternate supplier, you need to update your plan to downgrade the risk because you have taken the appropriate steps to mitigate it and eliminate the identified vulnerability.
Qualifying a Secondary Supplier
When qualifying a secondary supplier you may want to consider choosing a supplier in a different geographic location. There may be geographical situations or circumstances that could affect the ability of the original supplier to supply materials in a timely manner. This concept could also be applied not only to secondary suppliers of a raw material but also to suppliers of the final pharmaceutical product.
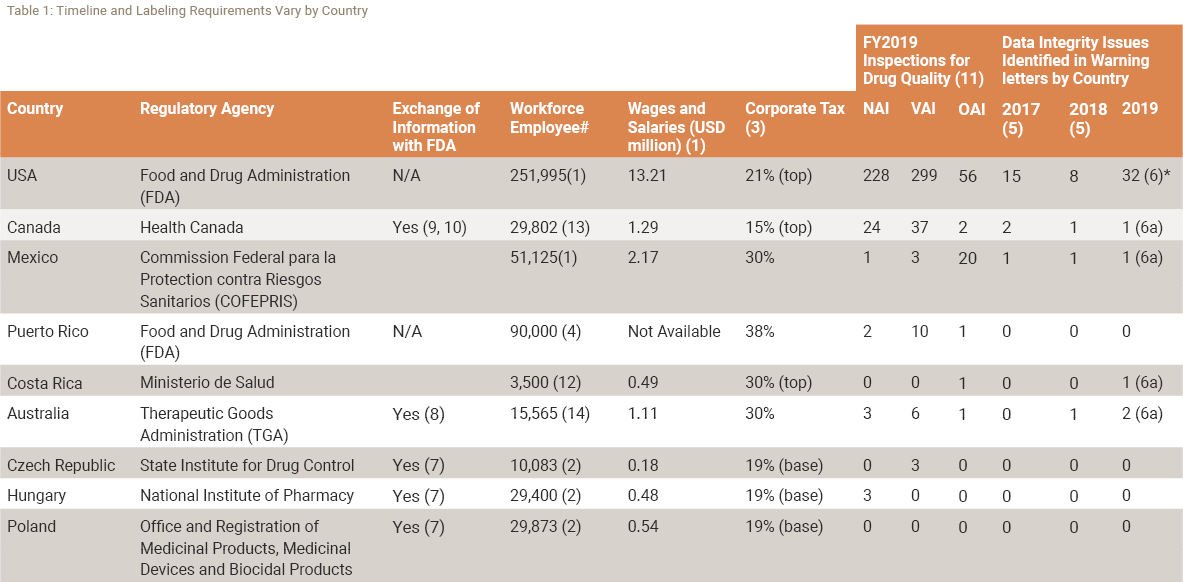
When choosing a new supplier in a different geographical location it will be important to understand the capabilities of the new region. The following Table demonstrates some of the information you will want to assess.
The chart identifies the Regulatory Agency in charge of the region you might be considering. It identifies the estimated number of employees currently in the industry and results of 2019 drug quality inspections (NAI, VAI, and OIA). Issues on Data Integrity are also tied to warning letter citations.
Geographic Risk Management
The decision to qualify a new location is a difficult one but with the proper information, it can be done. Quality risk management plans are important because they help improve a company’s ability to provide a quality product to patients. They are contingency plans with identified actions that help to ensure a continuous supply of product to the market. Further, the risk management plan is designed to accelerate products that are safe, effective, and available.
They are dynamic documents that require integration into and data inputs from all departments in order to be successfully implemented at a company, require integration into and data inputs from all departments in order to be successfully implemented at a company.
To begin the Regulatory Compliance Associates scoping process today, please enter your information in the blue form below and click the submit button at the bottom of the webpage. You may also email us at [email protected].
Connect with RCA Today
Contact us to learn more about our regulatory compliance experts and how they can help



















