Case Studies
Accelerating a Product Launch While Creating a Quality System

Client
Fortune 500 manufacturer of consumer products in need of quality management system support.
Industry
Medical Device
Business Challenge
Regulatory requirements for Design Master Record and Design History File for product launch of a new medical device.
Project Timeline
3 months
About QMS for Medical Devices
The FDA Quality System (QS) regulation applies to finished device manufacturers who intend to commercially distribute medical devices. Design controls for new product development are part of a Quality System. Within Design controls is the design history file (DHF) which demonstrates the product design was developed in accordance with the approved design plan and the requirements of the regulations (21 CFR Part 820.30).
Client Challenge
A fortune 500 consumer products company was planning new product development for their initial foray into the medical device space. Their accomplished design team was introducing an innovative therapy for home use. Recognizing that risk management and speed to market would be critical for early adoption and success, the parent company decided to create a new subsidiary to develop and launch the product. The seasoned Quality Assurance (QA) management elected to stay with the fortune 500 enterprise instead of joining the subsidiary, whereas some of the early and mid-careerists were attracted to the start-up venture. The subsidiary realized they had staff to implement but lacked QA leadership and deep medical device expertise.
Product Development Process
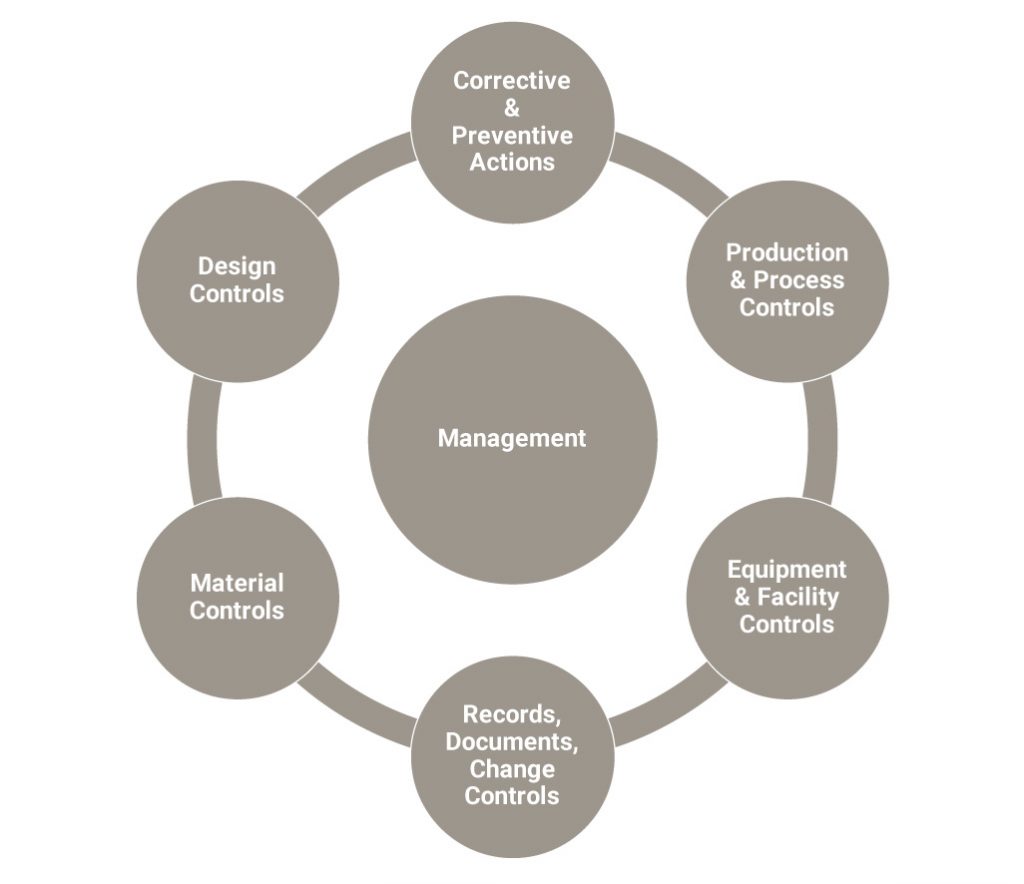
Regulatory Compliance Associates® Inc. (RCA) was engaged to recommend and establish a compliant Quality System. Thinking it would be faster than starting afresh, the subsidiary had initially proposed adoption of the parent company’s QS. RCA evaluated the legacy QS and found it lacking the medical device requirements. Over the course of 90 days, RCA developed a QS including the following elements:
- Management representative role
- Risk Management file and activities
- Design History File including documentation of user needs and design inputs, design outputs, design verification and validation protocols and reports, and design reviews
- Drafting and implementing QS standard operating procedures
- Document Control and Quality Records processes and records
- Device Master Record
- Design Change process and records
- Manufacturing Design transfer plans
To accomplish the resulting QS system, see figure 1 below, RCA provided the subsidiary with experts in product design, including mechanical electrical, software and user advocates, as well as experts in quality systems and design history files.
Results
With RCA’s outsourced QA expertise and their implementation staff, the subsidiary was able to launch the product, comply with all regulations, pass regulatory audits, and implement their Quality System without hiring expensive executives. Additionally, some of the subsidiary staff saw RCA as mentors, used this opportunity to enrich their skills, and increased their contribution to the organization. Over time, there were some internal promotions within the QA team as RCA helped create a culture of grooming and promoting from within.
The subsidiary continued their engagement with RCA to enrich their QS with subsequent phases including transitional quality process control, Product Complaint Handling, CAPA, Quality Manual, Regulatory Reporting, Internal Audits, Post Production and Management Review. As these milestones were achieved and as company sales grew, the subsidiary became attractive to investors and was sold to an established medical device company.