Blog
Understanding and Applying the Streamlined QMS Framework
In today’s rapidly evolving life sciences landscape, pharmaceutical and biologic companies are increasingly venturing into the world of combination products. These innovative therapies, which integrate drugs or biologics with medical devices, offer enhanced patient convenience, improved therapeutic outcomes, and a competitive edge in the marketplace. However, entering this space requires a strategic expansion of your existing Quality Management System (QMS) to meet regulatory expectations. That’s where the streamlined approach comes in.
Why a Streamlined QMS Approach Matters
The FDA’s implementation of 21 CFR Part 4 and the harmonization with ISO 13485 have paved the way for a simplified yet robust method for integrating device regulations into an existing pharmaceutical QMS. Known as the “streamlined approach,” this method allows companies to incorporate only six key elements from the medical device QMS framework, rather than overhauling their entire system. This is a significant advantage for organizations looking to enter the combination product market without disrupting their current operations.
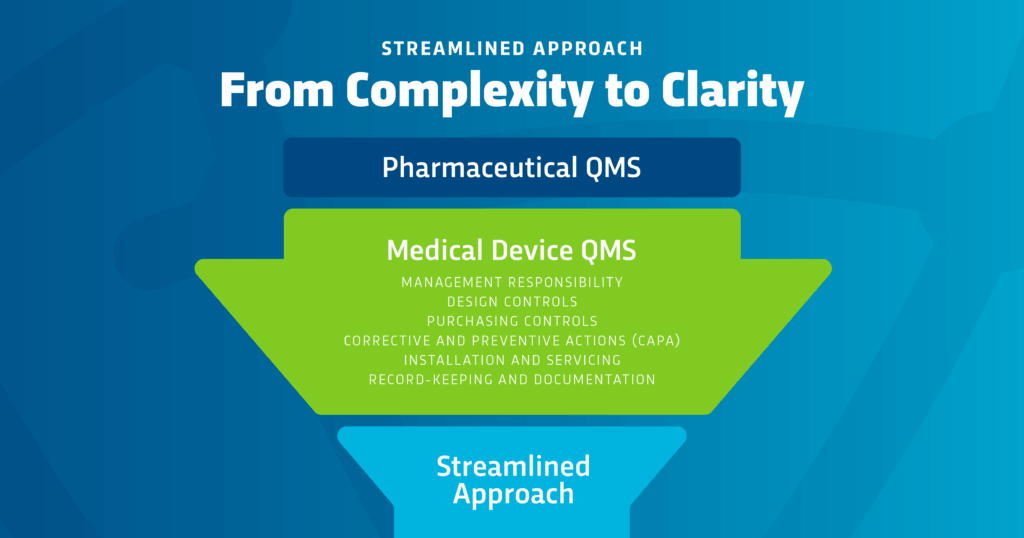
The Six Core Elements of FDA’s Streamlined Approach
- Management Responsibility: Establishing executive oversight and accountability for the expanded QMS.
- Design Controls: Implementing structured documentation and testing protocols for the device component.
- Purchasing Controls: Ensuring supplier qualification and ongoing performance monitoring.
- Corrective and Preventive Actions (CAPA): Addressing systemic issues through root cause analysis and effectiveness checks.
- Installation and Servicing: Defining procedures for proper setup and maintenance of device components.
- Recordkeeping and Documentation: Maintaining comprehensive records that demonstrate compliance.
For more details, refer to the FDA’s CGMP Companion Guidance for Combination Products.
Key Benefits for Pharmaceutical and Biologic Companies
- Efficiency: Focused integration minimizes disruption and accelerates time-to-market.
- Compliance Confidence: Aligns with FDA and international standards, reducing regulatory risk.
- Scalability: Easily adaptable to different product types and organizational sizes.
- Cost-Effectiveness: Avoids the need for a full QMS rebuild, saving time and resources.
Preparing for FDA’s 2026 QMSR Transition
With the FDA’s Quality Management System Regulation (QMSR) set to take effect on February 2, 2026, aligning your QMS with ISO 13485 is no longer optional—it’s essential. The streamlined approach offers a clear, actionable path to compliance that supports innovation and growth in the combination product space.
Partner with RCA for End-to-End Compliance Support
Navigating the complexities of combination product compliance doesn’t have to be overwhelming. Regulatory Compliance Associates® (RCA) brings decades of experience and a team of over 500 global experts to help you expand your QMS with confidence. Whether you need SOP development, training, or full-scale implementation support, RCA is your trusted partner in regulatory, quality, and compliance excellence.
Ready to streamline your QMS for combination product success?
Contact RCA today to schedule a consultation and take the first step toward regulatory readiness and market leadership.



















