IVDR Regulation for EU
IVDR meaning and IVD Regulation
Reasoning for IVDR Regulation
Previously, the MDD regulation and framework for the medical device industry had been in place for over 20 years. It came under harsh criticism in the media and political arena in particular after findings of the French health authorities. A French manufacturer (Poly Implant Protese, PIP) used industrial silicone instead of medical grade silicone for manufacture of breast implants over several years.
IVDR Performance Evaluation
The primary driver of IVDR regulation is several weaknesses that have undermined the objectives of the three medical device directives. Further, medical device companies and medical device safety in general has become more important globally.
Free circulation within the internal market was identified in public consultation held by Commission in 2008, and again in 2010. In addition, the new IVDR regulations aims at overcoming these gaps while maintaining the objectives of the legal framework.
MDD Issues
Regulated companies previously worked with over 70 designated notified body members under the three medical device directives. Simultaneously, the Notified Body Operations Group (NBOG) was designed to improve the overall performance of notified bodies in the medical devices sector.
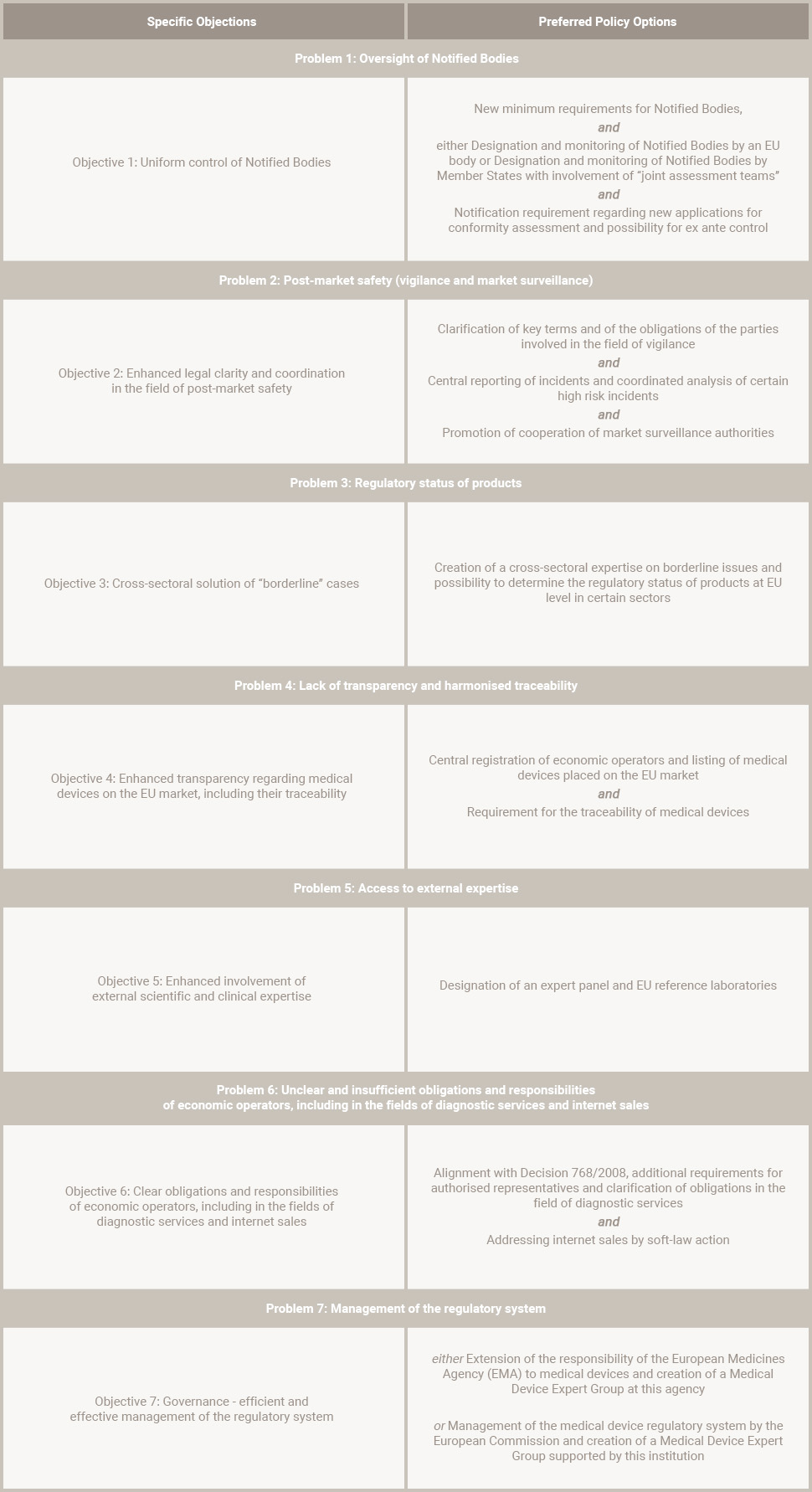
Oversight of Notified Bodies
Authorities and medical device manufacturers reported significant differences in the designation and monitoring of the notified bodies. For instance, the quality and depth of the conformity assessment came under intense scrutiny.
Factory Acceptance Inspection
Unannounced factory inspections or product checks led to an uneven level of protection of patients and users. Accordingly, there were noticeable differences between how global regulatory agencies and notified bodies reviewed manufacturers of similar products.
Post-Market Safety
Experience with application of the vigilance system and other legal instruments available to Member States (e.g., safeguard clauses) also came under scrutiny.
As a result, industry reports demonstrated that national competent authorities did not have the necessary global market research for decision making. information available to react similarly to the same problems. which begs the question of a harmonized level of protection.
Transparency
No exact data exist as regards the medical devices placed on the European market. Several Member States have set up their own electronic tools, and multiple registration requirements in individual member states. This places an administrative burden on manufacturers and authorized representatives to market a product in different Member States.
Traceability
Some have started imposing traceability requirements on economic operators. However, the national systems are not compatible with each other and do not allow traceability across borders which is necessary for an EU-wide high level of patient safety.
External Expertise
External experts are currently not involved in the regulatory process in a structured way.
EU Regulatory System
The management of the regulatory system at EU level has shown weakness which have been reported by various interested parties; it is considered as not sufficiently efficient and effective.
There is no legal basis in the medical devices directives to ensure an overview of the situation at EU level and appropriate coordination between Member States.
Technical Support
There is a lack of technical, scientific, and logistic support leading to a lack of uniform application of the rules and of common reactions in European market. The demarcation between the medical device directives and other regulatory frameworks is not always clear which leads to the application of different legal regimes.
Obligations of economic operators are currently not clear or covered by the directives at all.
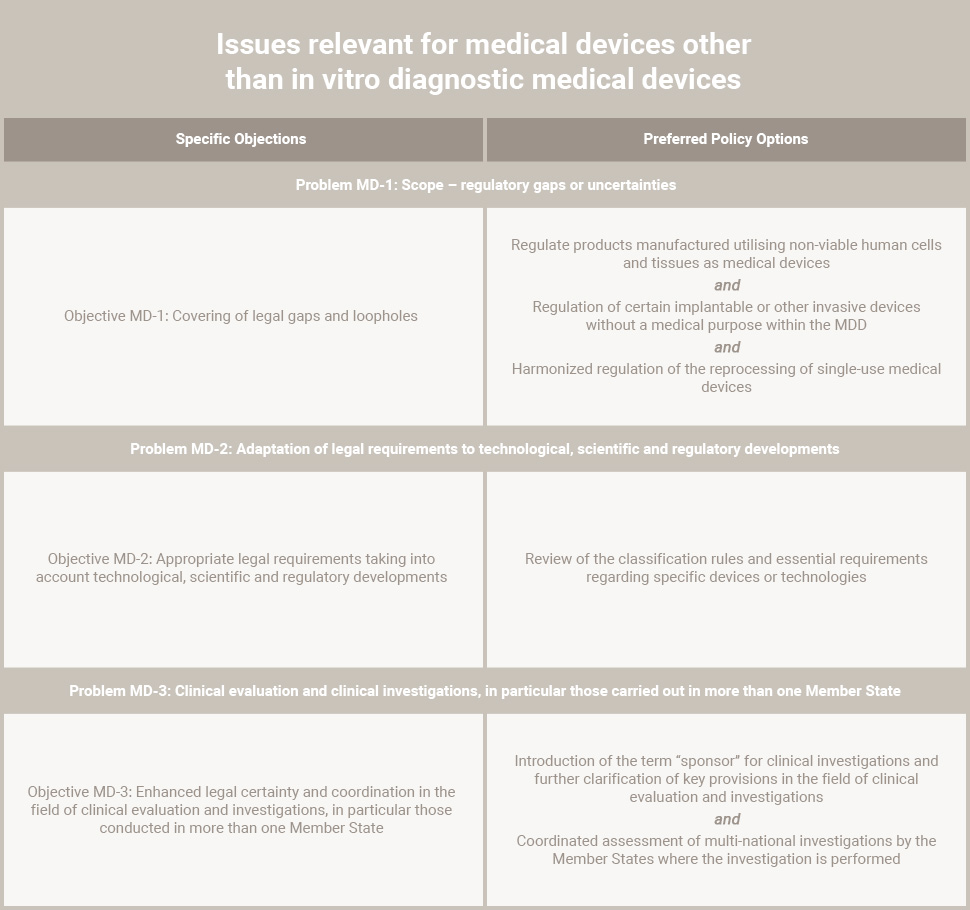
Specific Issues
Regulatory gaps exist with regard to certain products. For example, products manufactured utilizing nonviable human tissues or cells, implantable or other invasive products without a medical purpose. Also, the reprocessing of single-use devices is not regulated by EU legislation.
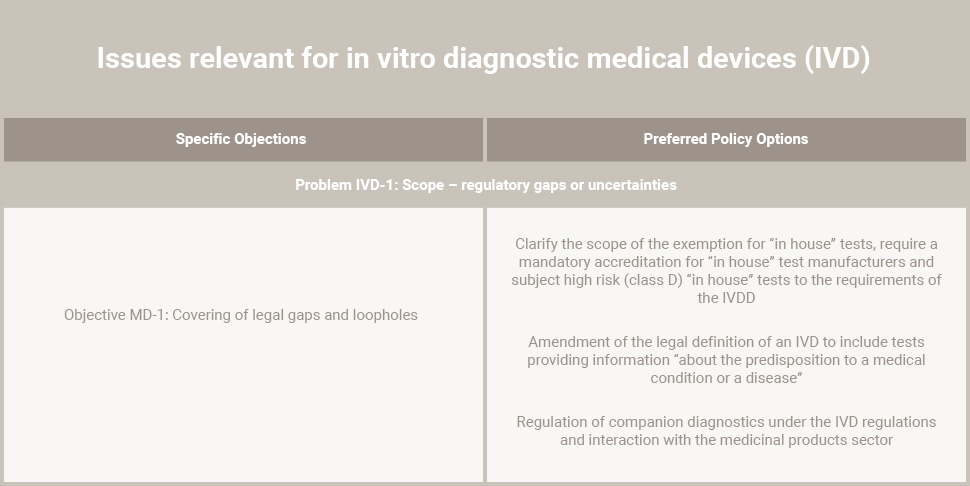
IVD’s “in-house” tests are currently exempt from the IVDR regulation.
IVD classification
Another important issue, classification of IVD’s: current approach for IVD classification is different from the classification approach taken from other medical devices and IVDR regulation.
Requirements of the IVDR regulation, needs to be adapted to technological, scientific, or regulatory developments.
Risk Classification
For medical devices, some legal provisions, such as essential requirements and criteria for risk classification of devices, do not sufficiently reflect the technological and scientific developments.
EU legislation currently does not make provision for any coordination between Member States. Further, the assessment of applications for clinical investigations is still individually conducted in more than one Member State.
Clinical Investigations
Moreover, this revision provides the opportunity to align the provisions regarding clinical investigations on medical devices, where appropriate, with the recently adopted Proposal for a Regulation on clinical trials on medicinal products for human use.
Proposed Revision
The current medical device directives are based on the treaty provisions regarding the establishment and functioning of the internal market (Article 114 TFEU). The Libson Treaty has added a legal basis in the area of public health for the adoption measures setting high standards of quality and safety of medical products (Article 168(4)(c) TFEU).
The proposed revision of the existing directives will integrate the modification of the Libson Treaty, to improve the level of protection of public health for all European parties and users.
Harmonized rules and procedures will allow manufacturers and Subject Matter Experts (SMEs) to reduce costs related to national regulatory differences, while ensuring a high and equal level of safety.
Moreover, this revision provides the opportunity to align the provisions regarding clinical investigations on medical devices. Where appropriate, this supports the adopted Proposal for a Regulation on clinical trials on medicinal products for human use.
IVDR regulation
The IVDR regulation revision pursues three overall objectives:
- To ensure a high level of protection of human health and safety
- To ensure the smooth functioning of the internal market
- To provide a regulatory framework which is supportive for innovation and the competitiveness of the European Medical device industry
In addition, several specific objectives related to individual problems identified.
The new legislation will be in the form of regulations – which are directly enforceable in Member States – in contrast to the current regime, which is based on Directives.The following table indicates the preferred policy options in the field of medical devices:
The following tables indicate the preferred policy for in vitro diagnostic devices:
Summary of IVDR Regulation Revision
The regulation proposal has been ratified by the European Parliament and council. The major difference is that the new legislation will be in the form of regulations. Additionally, these are directly enforceable in Member States—in contrast to the current state based on Directives.
The new regulations cover a wide range of products for the assessment of medical interventions. As it currently stands, the new regulatory environment will include the following:
- Greater transparency for patients—in particular those taking part in clinical trials.
- Manufacturers and importers now register themselves and devices on the EU market in a central databaseknown as EUDAMED — the European databank on medical devices.
- Manufacturers of medical devices will have to fit their products with a unique device identification to ensure traceability.
- Reinforced rules governing clinical evaluation throughout the life of the device.
- Introduction of the ‘sponsor’. This new requirement for medical device manufacturers means a single ‘qualified person’ is responsible for regulatory compliance.
- New rules for the reprocessing of single-use medical devices to make them suitable for further use.
- An EU portal where manufacturers would report serious incidents and corrective actions taken to reduce the risk of recurrence.
- A post-market surveillance system detailing device manufacturers’ responsibilities for follow-up of the quality, performance and safety of devices. This includes annual periodic safety update reports (PSURs).
- A tightening of the rules for the designation of notified bodies. To illustrate, the monitoring of assessment activities by national competent authorities and cooperation between competent authorities. The new rules would also give notified bodies the right to carry out spot checks of unannounced factory inspections.
- Extension of the scope of medical device regulation to cosmetic / aesthetic devices as well as ‘ingested products’.
- The introduction of a new expert group: the Medical Device Coordination Group (MDCG). This group has the power to review Notified Body assessments of high-risk devices before approved for market.
About RCA’s Medical Device Consulting Services
The regulatory compliance process surrounding the medical device industry involves a strict adherence to pre/post market information throughout a device’s life cycle. Even a single compliance issue you have can turn into a significant effect on your business. Regulatory Compliance Associates can help guide you through any stage of the medical device consulting process, with capabilities during product development through the regulatory clearance/approval of your product.
Our team of over 500 medical device consulting Experts — including former FDA officials and regulatory compliance leaders in the field of medical device regulation — will work with your company to create a quality assurance and regulatory compliance approach tailored to your products and regulatory needs. Regulatory Compliance Associates works with international Fortune 100 companies, venture capital start ups, and companies of all sizes and shapes. our compliance enforcement solutions for law firms include remediation for warning letters, FDA 483’s, import bans or consent decrees. Very few regulatory compliance services have the same regulatory compliance expertise in a variety of medical fields.
Cybersecurity
For medical device manufacturers, technology can be a double-edged sword. The innovative technologies that elevate the quality of life for patients can also be used to potentially undermine the organization using the device. The consequences can affect the device itself if Regulatory Compliance Associates medtech consultants do not implement good IoT cybersecurity and FDA cybersecurity protocols.
At Regulatory Compliance Associates, we offer a wide variety of services for medical devices security to help ensure that your product is protected from cyber-attacks. With a well-planned design, along with full visibility of product development and the supply chain, Regulatory Compliance Associates medical device consultant Experts can help strengthen your device’s cybersecurity. We partner with medical device companies in each phase of the design cycle, including protecting inputs from threat exposure and hardening outputs for regulatory compliance & FDA submission approval of your medical technology.
- SaMD Consulting
- Threat Modeling
- Proof of Concept
- Quality Assurance Services
- TIR 57 & TIR 97
- ISO 62304
- ISO 27001
Regulatory Affairs
Regulatory affairs is Regulatory Compliance Associates® backbone, and we handle more submissions in a month than many manufacturers do in a lifetime. Our regulatory compliance consulting Experts have experience working with the FDA, global regulatory bodies and / or agencies, and notified bodies worldwide. Therefore, you can count on us for in-depth and up-to-date insights which increase speed-to-market.
As a trusted regulatory affairs consultant, our FDA veterans and industry experts represent Regulatory Compliance Associates® as one of the top medical device consulting firms. We’re here to help you navigate the difficulties associated with new product submissions. Regulatory Compliance Associates® medical device consulting company has expertise in both the approval process and post-approval support.
- New Product Approval
- Post-Approval Support
- Outsourced Staffing
- EU MDR
- Combination Products
Compliance Assurance
Increasingly, life science companies are feeling the pressure of greater scrutiny by regulators, and responding by developing sustainable compliance strategies. Whether it’s preparing for an audit, developing a response to an FDA finding, or remediation to an adverse event, Regulatory Compliance Associates® can help.
Our network of over 500 medical device consultant & FDA, MHRA & EMA veterans are industry professionals offers a unique blend of expertise. This allows Regulatory Compliance Associates® to handle both simple and complex regulatory compliance challenges within medical device consulting companies.
- Gap Assessments
- Internal Audits
- Employee Training
- Notified Body Response
- Data Integrity
Quality Assurance
Regulatory Compliance Associates® Quality Assurance consulting includes quality system assessments, strategy, implementations, and identification of quality metrics to ensure continuous improvement, aligning with your business needs and goals. Each Regulatory Compliance Associates® medical device consultant is a quality expert with experience spanning major corporations and start-ups. We know firsthand how to achieve, maintain, and improve quality, and we excel in transferring this knowledge to your organization.
In the medical devices field, quality assurance (QA) is more than merely ensuring the quality of a finished product. You need the tools to monitor and regulate every process from the design of a new product to continued quality compliance as the device is sent to market. At Regulatory Compliance Associates®, we offer you the quality assurance services you need to monitor these processes and ensure quality compliance every step of the way.
With more than 20 years experience working with medical device consulting companies, Regulatory Compliance Associates® trusted medical device quality assurance consultant team is fully equipped to handle your unique QA needs.
- ISO13485
- 21 CFR 210
- 21 CFR 211
- Outsourced Staffing
- MDSAP
- Facility Validation
- Equipment Validation
- Quality Metrics
Remediation Services
Regulatory Compliance Associates® is widely recognized within medical device consulting companies & the life science industry for our remediation services & support. Regulatory Compliance Associates® ability to help companies successfully resolve complex regulatory challenges have a proven track record of success. Our medical device consulting services include significant experience with the development of responses to 483 Observations, Warning Letters, Untitled Letters and Consent Decrees.
- Regulatory Action
- Regulatory Compliance
- Regulatory Enforcement
- Warning Letter
- 483 Observation
- Oversight Services
Our value goes beyond the initial response by helping companies successfully execute their action plans, develop an improved compliance culture tailored to the needs of their business, and ultimately move beyond the regulatory action to emerge as a stronger business. We negotiate difficult demands of remediation with insight and the clear advantage of our medical device consultant expertise and experience that makes partnering with Regulatory Compliance Associates® a competitive differentiator in the remediation space.
- Quality System
- Technical File
- Design History File
- Data Integrity
- cGMP
Strategic Consulting
Whether it’s a strategy, a technical plan, or project, Regulatory Compliance Associates® medical device consultancy can help ensure a successful project. Regulatory Compliance Associates® medical device strategy consulting can deliver your project on time, on budget, and you’re never embroiled in a costly mistake.
Our medical device consultant Experts are industry Experts are here to provide the unique insight you need before an M&A deal, through a staffing crisis and in every area of your product’s development and life cycle. As the trusted medical device manufacturing consultants of thousands of companies around the world, we have the knowledge and expertise needed to deliver exceptional results to your business — no matter your size or unique needs.
- Manufacturing Optimization
- Product Lifecycle Management
- Mergers & Acquisitions (M&A)
- Due Diligence
- Device Vigilance
- Risk Management Plan
- Product Complaints
- Medical Information
About Regulatory Compliance Associates
 Regulatory Compliance Associates® (RCA) provides medical device consulting to the following industries for resolution of life science challenges:
Regulatory Compliance Associates® (RCA) provides medical device consulting to the following industries for resolution of life science challenges:
- Life Sciences
- Pharmaceutical
- Biologic & Biotechnology
- Sterile compounding
- Medical device
- Lab Testing
We understand the complexities of running a life science business and possess areas of expertise that include every facet of R&D, operations, regulatory affairs, quality, and manufacturing. We are used to working on the front lines and thriving in the scrutiny of FDA, Health Canada, MHRA and globally-regulated companies.
As your partners, Regulatory Compliance Associates can negotiate the potential minefield of regulatory compliance and regulatory due diligence with insight, hindsight, and the clear advantage of our unique expertise and experience.
- Founded in 2000
- Headquartered in Wisconsin (USA)
- Expertise backed by over 500 industry subject matter experts
- Acquired by Sotera Health in 2021
About Sotera Health
The name Sotera Health was inspired by Soteria, the Greek goddess of safety, and reflects the Company’s unwavering commitment to its mission, Safeguarding Global Health®.
Sotera Health Company, along with its three best-in-class businesses – Sterigenics®, Nordion® and Nelson Labs®, is a leading global provider of mission-critical end-to-end sterilization solutions and lab testing and advisory services for the healthcare industry. With a combined tenure across our businesses of nearly 200 years and our industry-recognized scientific and technological expertise, we help to ensure the safety of over 190 million patients and healthcare practitioners around the world every year.
We are a trusted partner to more than 5,800 customers in over 50 countries, including 40 of the top 50 medical device companies and 8 of the top 10 pharmaceutical companies.
Commitment to Quality
Our Certificate of Registration demonstrates that our Quality Management System meets the requirements of ISO 9001:2015, an internationally recognized standard of quality.
To begin the Regulatory Compliance Associates® scoping process today, please enter your information in the blue form below and click the submit button at the bottom of the webpage.
References
European Union (2012), Executive Summary of the Impact Assessment on the Revision of, the Regulatory Framework for Medical Devices. SWD(2012) 274 Final. Brussels. Resource.